Answer: The partial pressure of carbon dioxide is 712.8
Step-by-step explanation:
Dalton's law of partial pressure states that the total pressure of the system is equal to the sum of partial pressure of each component present in it.
To calculate the partial pressure of carbon dioxide gas, we use the law given by Dalton, which is:
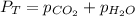
We are given:
Total pressure,
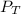 = 734 torr
= 734 torr
Vapor pressure of water,
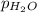 = 21.2 torr
= 21.2 torr
Putting values in above equation, we get:
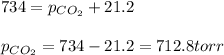
Hence, the partial pressure of carbon dioxide is 712.8