Answer:
(a) 65.04%
(b) 16.91%
Solution:
As per the question:
At inlet:
Pressure of the compressor, P = 140 kPa
Temperature, T =
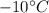 = 263 K
= 263 K
Isentropic work, W = 700 kPa
At outlet:
Pressure, P' = 700 kPa
Temperature, T' =
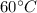 = 333 K
= 333 K
Now, from the steam table;
At the inlet , at a P = 700 kPa, T =
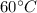 :
:
h = 243.40 kJ/kg, s = 0.9606 kJ/kg.K
At outlet, at P = 140 kPa, T =
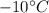 :
:
h' = 296.69 kJ/kg, s' = 1.0182 kJ/kg.K
Also in isentropic process, s =
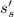 and
and
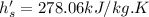 at 700kPa
at 700kPa
(a) Isentropic efficiency of the compressor,
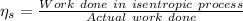
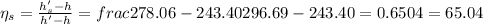
(b) The temperature of the environment,
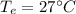 = 273 + 27 = 300 K
= 273 + 27 = 300 K
Availability at state 1,
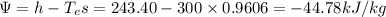
Similarly for state 2,
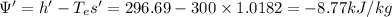
Now, the efficiency of the compressor as per the second law;
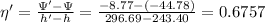 = 67.57%
= 67.57%