Answer:
The final gauge pressure is 70 kPa
Solution:
As per the question:
Gauge pressure, P = 50 kPa = 50000 Pa
Now,
The specific volume is given by:
v =
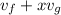
where
x = 0.75
Using saturated vapor- pressure table,
At a pressure of 50 kPa
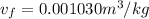
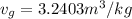
Thus
v_{g} = 0.001030 + 0.75\times 3.2403 = 2.431255
Now,
The final gauge pressure is the pressure (saturation) which corresponds to the value of
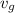
Thus at
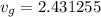
Final Pressure, P' = 70 kPa