Answer:
158.57 kJ
Step-by-step explanation:
We can assume air is an idela gas and the ideal gas law applies.
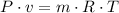
And we know that:
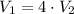
WE can assume the process is isothermal
Therefore the work:
W=mRTln(V₂/V₁)
We can determine the constant C as:
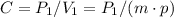
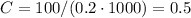
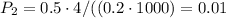
P₂ is 0.01 kPa
V₂=0.005
V₁=50
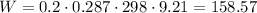
The total work is 158.57 kJ