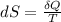Answer:
a. all systems move toward increased entropy
Step-by-step explanation:
According to the second law of thermodynamics, in an isolated system the total entropy can never decrease over time. The entropy may remain constant under ideal conditions but can never decrease.
For closed system reversible process
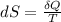
Where,
ds = Change in Entropy
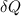 = Change in heat
= Change in heat
T = Temperature
For closed system irreversible process
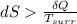
For quasistatic irreversible process without composition change