Answer:
Absolute pressure , P(abs)= 433.31 KPa
Step-by-step explanation:
Given that
Gauge pressure P(gauge)= 50 psi
We know that barometer reads atmospheric pressure
Atmospheric pressure P(atm) = 29.1 inches of Hg
We know that
1 psi = 6.89 KPa
So 50 psi = 6.89 x 50 KPa
P(gauge)= 50 psi =344.72 KPa
We know that
1 inch = 0.0254 m
29.1 inches = 0.739 m
Atmospheric pressure P(atm) = 0.739 m of Hg
We know that density of Hg =
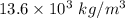
P = ρ g h
P(atm) = 13.6 x 1000 x 9.81 x 0.739 Pa
P(atm) = 13.6 x 9.81 x 0.739 KPa
P(atm) =98.54 KPa
Now
Absolute pressure = Gauge pressure + Atmospheric pressure
P(abs)=P(gauge) + P(atm)
P(abs)= 344.72 KPa + 98.54 KPa
P(abs)= 433.31 KPa