Answer:
entropy, s = 4.4076 x
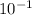 KJ/kg-K
KJ/kg-K
Step-by-step explanation:
Given
Pressure, P = 4 MPa
Enthalpy, h = 30.96 KJ/kg
Therefore using steam table for water, we get
entropy at pressure, P = 4 MPa and Enthalpy, h = 30.96 KJ/kg is
entropy, s = 4.4076 x
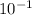 KJ/kg-K
KJ/kg-K
Therefor, the answer is entropy, s = 4.4076 x
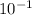 KJ/kg-K
KJ/kg-K