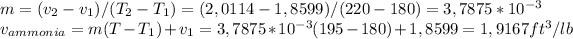Answer:
v_water=1,0432e-3 m3/kg
v_r22=0,801412 ft3/lb
v_ammonia=1,9167 ft3/lb
Step-by-step explanation:
To calculate the specific volumes of pure substances like water, refrigerant 22 and Ammonia, the respective properties tables are needed. In my case, I took the values from the following reference: Fundamentals of Engineering Thermodynamics 7th Edition, Moran, Shapiro et al.
In the case of the water, the specific volume v_f can be taken from the saturated water (liquid-vapor), pressure table (Table A-3). Within the table we look for the pressure value and then the v_f specific volume. The values are the following ones:
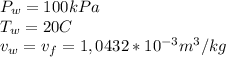
Speaking of the refrigerant 22 is a little bit more complicated because of the quality value. First we have identify the following values for the 40 lbf/in2 pressure value (Table A-8E):
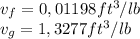
Then the specific volume can be calculated as follows:
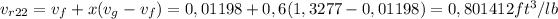
Regarding the Ammonia, the saturation temperature of this substance is 96,31 °F which means that at 195 °F, we would have superheated ammonia vapor. As the values on the table for the 20 lbf/in2 pressure, are not available at 195 °F we have to look for the two values that the suggested temperature is between (In this case 180 and 220 °F). The following values, for the 20 lbf/in2 pressure value, are needed (Table A-15E):
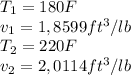
Then the specific volume can be calculated as follows: