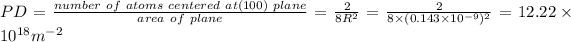Answer:
PD will be
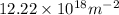
Step-by-step explanation:
We have given radius of aluminum r = 0.143 Nm
Plane is given as (100)
For (100) plane there is one atom at every one of the four 3D square corners, every one of which is imparted to four adjoining unit cells, while the middle atom lies totally inside the unit cell. Therefore, there is the proportionality of 2 particles related with this FCC (100) plane.
The planar section represented in the above figure is a square, wherein the side lengths are equal to the unit cell edge length,
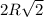 and thus, the area of this square is just =
and thus, the area of this square is just =
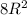
The planer density for the plane is