Answer: 42 moles
Step-by-step explanation:
The balanced chemical reaction for combustion of 1 mole of glucose is:
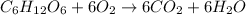
Energy released during combustion of 1 mole of glucose = 2870 kJ
It is also given that:
Energy required to form 1 mole of compound X = 67.5 kJ
This 67.5 kJ of energy is used to form = 1 mole
2870 kJ of energy is used to form =
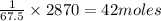
Thus 42 moles of the compound X could theoretically be generated.