Step-by-step explanation:
The given data is as follows.
 = 25 ml,
= 25 ml,
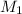 = 0.10 M
= 0.10 M
 = x ml =
= x ml =
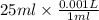 ,
,
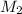 = 0.650 M
= 0.650 M
It is known that relation between molarity and volume of two solutions is as follows.
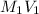 =
=
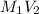
 =
=
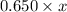
x = 0.00385 L
or, = 3.85 ml
Since, this volume is closest to 4 ml so, we have to use a 2 ml pipette two times and then we have to put it in a 25 ml volumetric flask.
Hence, calculate the overall concentration as follows.
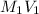 =
=
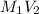
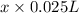 =
=
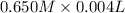
x = 0.104 M
Therefore, it is about 3.8% error that is lower than the required error.