Step-by-step explanation:
The given data is as follows.
Mass of mixture = 0.3471 g
As the mixture contains oxalic acid and benzoic acid. So, oxalic acid will have two protons and benzoic acid has one proton.
This means oxalic acid will react with 2 moles of NaOH and benzoic acid will react with 1 mole of NaOH.
Hence, moles of NaOH in 97 ml =
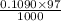
=
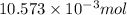
Moles of HCl in 21.00 ml =
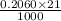
=
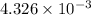 mol
mol
Therefore, total moles of NaOH that reacted are as follows.
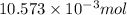 -
-
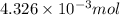
=
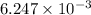 mol
mol
So, total 3 mole of NaOH will react with 1 mole of mixture. Therefore, number of moles of NaOH reacted with benzoic acid is as follows.
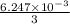
=
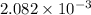 mol
mol
Since, molar mass of NaOH is 40 g/mol. Therefore, calculate the mass of NaOH as follows.
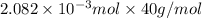
=
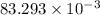 g
g
= 0.0832 g
Whereas molar mass of benzoic acid is 122 g/mol.
Therefore, 40 g NaOH = 122 g benzoic acid
So, 0.0832 g NaOH =
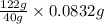
= 0.253 g
Hence, calculate the % mass of benzoic acid as follows.
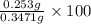
= 73.10%
Thus, we can conclude that mass % of benzoic acid is 73.10%.