Answer : The correct option is, (b) 202 g
Explanation :
The given set of balanced reactions are:
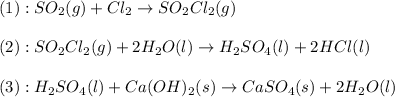
First we have to calculate the moles of
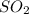 .
.
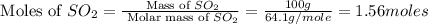
Now we have to calculate the moles of
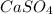 .
.
From the given set of balanced reactions we conclude that,
As, the mole ratio of
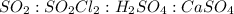 is, 1 : 1 : 1 : 1
is, 1 : 1 : 1 : 1
So, the moles of
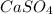 = moles of
= moles of
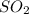 = 1.56 moles
= 1.56 moles
Now we have to calculate the mass of
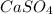 .
.
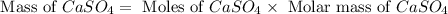
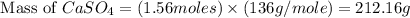
As we are given that the process is 95.0 % efficient that means the amount we calculated is recovered.
Mass of
 =
=
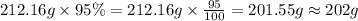
Therefore, the mass of
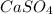 produced is 202 grams.
produced is 202 grams.