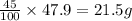Answer:a) Mass of
 in mixture = 26.3 grams
in mixture = 26.3 grams
b) Mass of
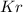 in mixture that can be recovered= 21.5 grams
in mixture that can be recovered= 21.5 grams
Step-by-step explanation:
Given :
Total Pressure = pressure of krypton + pressure of carbon dioxide = 0.751 atm
pressure of krypton = 0.231 atm
Thus pressure of carbon dioxide = 0.751 - 0.231 =0.52 atm
As we know:
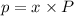
where,
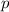 = partial pressure
= partial pressure
 = total pressure = 0.751 atm
= total pressure = 0.751 atm
 = mole fraction
= mole fraction
For

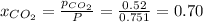
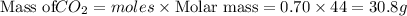
For
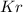
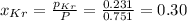
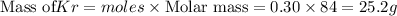
Total mass = Mass of
 + Mass of krypton = 30.8 + 25.2 = 56 g
+ Mass of krypton = 30.8 + 25.2 = 56 g
Percentage of
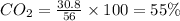
a) Thus Mass of
 in mixture =
in mixture =
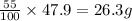
Percentage of
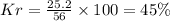
b) Thus Mass of
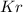 in mixture that can be recovered=
in mixture that can be recovered=