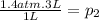Answer:
The resulting pressure is 4.2 atm. Since it's an ideal gas we use the ideal gas equation to calculate the final pressure.
Step-by-step explanation:
p.v=n.R.T
This applies to both initial and final conditions of the gas, since the temperature is held constant:
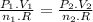
And using this equation we find the value of pressure: