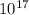Answer:
2.0*
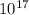
Step-by-step explanation:
Let us calculate total moles (air + CO)
moles (n) = PV /RT
P = 755 torr/760 = 0.9934 atm
V = 1.00 L
R = gas constant
T = 23°C + 273 = 296 K
So,
total moles (n) = 0.9934 x 1.00/0.0821 x 296 = 0.041 moles
mole fraction of CO = 8.1 x
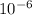
moles of CO =8.1 x
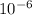 *0.041
*0.041
=3.31 x
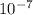 moles
moles
number of molecule of CO =3.31 x
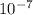 *(6.0233x
*(6.0233x
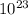 )
)
= 2.0*