Answer:
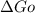 for couple reaction is -16.7 kJ/mol
for couple reaction is -16.7 kJ/mol
Step-by-step explanation:
Given data:
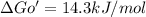 for glucose conversion to glucose 6 phosphate
for glucose conversion to glucose 6 phosphate
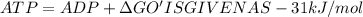
from above data we have following reaction
Glucose = Glucose 6 phosphate
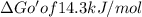
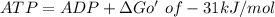
when coupling both reaction we have
Glucose + ATP = Glucose 6 phosphate + ADP
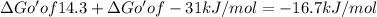
therefore
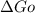 for couple reaction is -16.7 kJ/mol
for couple reaction is -16.7 kJ/mol