Answer:
Percentage of error is 67.2%
Step-by-step explanation:
from table A-1 of thermodynamics " gas constant and critical point properties" obtain CO_2 properties
from table
critical temperature
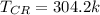
critical pressure
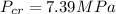
Gas constant R = 0.188 kJ/kg -K
From ideal gas equation we have
PV = RT
solving of v we have
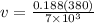
v = 0.01020 m^3/kg
Reduced Pressure
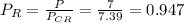
reduced temperature
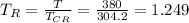
obtained compressibility factor (z) from chart between reduced temperature and reduced pressure.
from
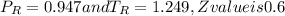
so we have
Pv = ZRT
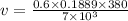
v = 0.00615 m^3/kg
percentage of error is
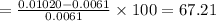 %
%