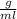The density of the metal object=6.0
Given:
Volume of the metal object=1.5ml
Mass of the metal object=9.0g
To find:
Density of the metal object
Step by Step Explanation:
Solution:
According to the formula, Density of the metal object can be calculated as
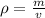
Where, m=mass of the metal object
 =density of the metal object
=density of the metal object
v=volume of the metal object
We know the values of v=1.5ml and m=9.0g
Substitute these values in the above equation we get
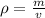
 =9.0/1.5
=9.0/1.5
=6.0
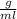
Result:
Thus the density of the metal object is 6.0