Answer:
-262.58 kJ
Step-by-step explanation:

The enthalpy represents the energy that is either absorbed or released during a chemical reaction.
We see that the chemical reaction that we have is balanced and 1 mol of calcium carbonate was formed. During this process -69.1 kJ or energy was released because it has a negative sign. We can say that the enthalpy changes is -69.1kJ per every mol of calcium carbonate formed.
Using a simple rule of three we can get the enthalpy change when 3.8 mol of
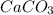 are formed.
are formed.
-69.1 Kj --- > 1 mol of
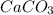
X --- > 3.8 mol of
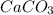
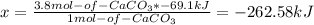
So, the energy released when 3.8 mol of calcium carbonate are formed is -262.58 kJ.