Answer:
-329.9°M corresponds to 0°K.
Step-by-step explanation:
First we need to know the freezing and boiling points of the Methane in the Kelvin scale. Since the Kelvin scale is related to the Celsius scale by:
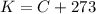
Then, the freezing and boiling points of the Methane are:
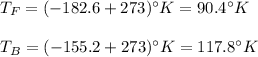
So, now we know the following relationship between Methane and Kelvin scales:
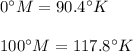
Now, if we think of that temperatures as the ordered pairs (0, 90.4) and (100, 117.8), we can obtain the equation of the line that contains that two points and find where the "y" (in this case, the temperature in Kelvin scale) is zero.
First, we find the slope:
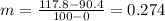
Now, we use the general formula of a line (remembering that y should be zero):
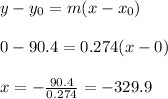
It means that the temperature of -329.9°M corresponds to absolute zero in Kelvin scale.