Answer:
0.075881 kJ
Step-by-step explanation:
The balanced equation for the reaction between
![Ba(OH)_(2){/tex] and HCl is:</p><p></p><p>[tex]2HCl + Ba(OH)_(2) --- > BaCl_(2) + 2H_(2)O]()
The enthalpy represents the energy that is either absorbed or released during a chemical reaction. It can be estimated experimentally using a calorimeter to measure the change in temperature after the reaction has occurred. The endpoint of the reaction is determined when there is no change in the temperature in the calorimeter.
With the data, we can use the next equation to estimate the heat (enthalpy) absorbed in this reaction.
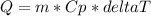
Where
Q is heat, m is mass of the reactants and Cp is the specific heat of the products and delta T is the change of temperature.
Mass of the reactants.
We know that molarity is
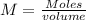
So
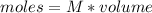
We can estimate the moles for each reactant as:
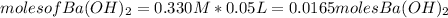
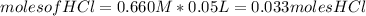
The molecular mass for each reactant is:
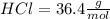
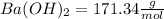
The mass for each one is
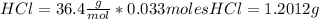
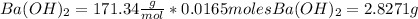
Total mass = 1.2012 g + 2.83 g = 4.0283 g
Cp is the one for the water
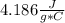
Delta T = 28.16 °C - 23.66 °C = 4.5 °C
Using the equation
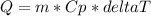
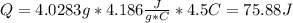
The heat absorbed in this reaction is 75.88 J or 0.075881 kJ