Answer:
The radius of tantalum (Ta) atom is
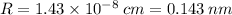
Step-by-step explanation:
From the Body-centered cubic (BBC) crystal structure we know that a unit cell length a and atomic radius R are related through
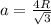
So the volume of the unit cell
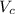 is
is
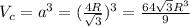
We can compute the theoretical density ρ through the following relationship
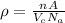
where
n = number of atoms associated with each unit cell
A = atomic weight
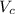 = volume of the unit cell
= volume of the unit cell
 = Avogadro’s number (
= Avogadro’s number (
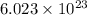 atoms/mol)
atoms/mol)
From the information given:
A = 180.9 g/mol
ρ = 16.6 g/cm^3
Since the crystal structure is BCC, n, the number of atoms per unit cell, is 2.
We can use the theoretical density ρ to find the radio R as follows:
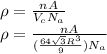
Solving for R
![\rho=(nA)/(((64√(3)R^3)/(9))N_(a))\\(64√(3)R^3)/(9)=(nA)/(\rho N_(a))\\R^3=(nA)/(\rho N_(a))\cdot (1)/((64√(3))/(9)) \\R=\sqrt[3]{(nA)/(\rho N_(a))\cdot (1)/((64√(3))/(9))}](https://img.qammunity.org/2020/formulas/chemistry/college/p1t6gfzs9lwbcb0vuf8zm7d8narh2ac10h.png)
Substitution for the various parameters into above equation yields
![R=\sqrt[3]{(2\cdot 180.9)/(16.6\cdot 6.023 * 10^(23))\cdot (1)/((64√(3))/(9))}\\R = 1.43 * 10^(-8) \:cm = 0.143 \:nm](https://img.qammunity.org/2020/formulas/chemistry/college/kupqen00b8u6sxqboviq1isuzbnnp5bup2.png)