Answer:
The concentration in the outlet water is 7.36x10-6 gNH3/gH2O
Step-by-step explanation:
We need to have the same units to do the operations so that we can substract the activity of the bacteria from the flow rate of gNH3/s for this we first need to know which is the flow of gNH3/s in the water flow rate for this we:
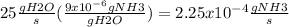
As you can see we used the concentration of NH3 in H2O to have the gNH3/s. Now we jst susbtract the activity of the baterias to the initial concentration of the water
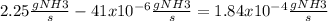
We can do this operation because the units are the same.
Now we just need to convert the 1.84x10-4 gNH3/s to concentration of gNH3/gH2O, for this we just neet to divide the concentration by the flow of the water (25gH2O/s)
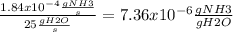
We cancel the units and when we realice the division we get that the outlet concentration of the water is 7.36x10-6gNH3/gH2O