Answer:
The minimum volume that the tank must have to inflate this balloon to its 1 m diameter is 174.53 L.
Step-by-step explanation:
Temperature of the gas = T = 25°C
Pressure of the helium gas in the tank connected to balloon =
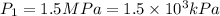
Let the minimum volume of the tank to inflate this balloon to its 1 m diameter be

Temperature of the gas after the valve is opened = T = 25°C
Pressure inside the balloon after the valve is opened =
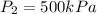
On opening the valve, helium gas starts moving towards balloon by changing its diameter to 1 m.
Diameter of balloon = d = 1 m
Volume of balloon after the valve is opened =

Radius of the balloon = r = d/2 = (1 m)/2 = 0.5 m

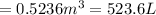
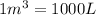
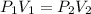 (Boyle's law)
(Boyle's law)
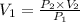
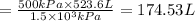
The minimum volume that the tank must have to inflate this balloon to its 1 m diameter is 174.53 L.