Step-by-step explanation:
As it is known that
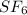 molecule is a non-linear molecule. Therefore, its isochoric heat capacity will be as follows.
molecule is a non-linear molecule. Therefore, its isochoric heat capacity will be as follows.
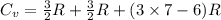
=
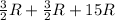
= 18 R
Also,
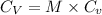
where,
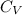 = molar heat capacity
= molar heat capacity
M = molecular mass
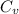 = specific heat
= specific heat
Hence, calculate the value of
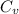 as follows.
as follows.
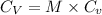
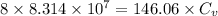
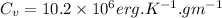
This means that value of isochoric specific heat is
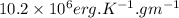 .
.
Yes, we have to assume ideal gas behavior because for ideal gas:
dU =
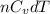
Whereas for real gases "
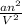 " has to be added here.
" has to be added here.