Answer:
For a: The activity coefficient of copper ions is 0.676
For b: The activity coefficient of potassium ions is 0.851
For c: The activity coefficient of potassium ions is 0.794
Step-by-step explanation:
To calculate the activity coefficient of an ion, we use the equation given by Debye and Huckel, which is:
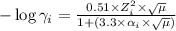 ........(1)
........(1)
where,
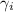 = activity coefficient of ion
= activity coefficient of ion
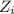 = charge of the ion
= charge of the ion
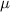 = ionic strength of solution
= ionic strength of solution
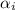 = diameter of the ion in nm
= diameter of the ion in nm
To calculate the ionic strength, we use the equation:
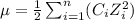 ......(2)
......(2)
where,
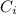 = concentration of i-th ions
= concentration of i-th ions
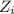 = charge of i-th ions
= charge of i-th ions
We are given:
0.01 M NaCl solution:
Calculating the ionic strength by using equation 2:
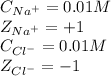
Putting values in equation 2, we get:
![\mu=(1)/(2)[(0.01* (+1)^2)+(0.01* (-1)^2)]\\\\\mu=0.01M](https://img.qammunity.org/2020/formulas/chemistry/college/vfrxyahndgxp9wmjk72zqim6aq1fvevxoe.png)
Now, calculating the activity coefficient of
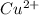 ion in the solution by using equation 1:
ion in the solution by using equation 1:
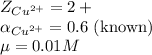
Putting values in equation 1, we get:
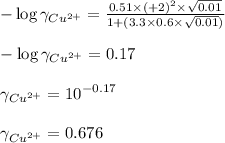
Hence, the activity coefficient of copper ions is 0.676
We are given:
0.025 M HCl solution:
Calculating the ionic strength by using equation 2:
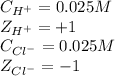
Putting values in equation 2, we get:
![\mu=(1)/(2)[(0.025* (+1)^2)+(0.025* (-1)^2)]\\\\\mu=0.025M](https://img.qammunity.org/2020/formulas/chemistry/college/dlv69oe824xxd1kyxnr9nv3cuhowul59cn.png)
Now, calculating the activity coefficient of
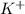 ion in the solution by using equation 1:
ion in the solution by using equation 1:
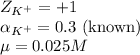
Putting values in equation 1, we get:
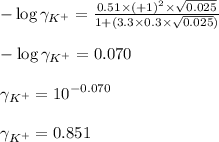
Hence, the activity coefficient of potassium ions is 0.851
We are given:
0.02 M
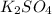 solution:
solution:
Calculating the ionic strength by using equation 2:
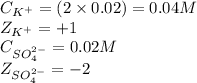
Putting values in equation 2, we get:
![\mu=(1)/(2)[(0.04* (+1)^2)+(0.02* (-2)^2)]\\\\\mu=0.06M](https://img.qammunity.org/2020/formulas/chemistry/college/b06rq4gr063bfok6bwopsv4oow85t5ls8q.png)
Now, calculating the activity coefficient of
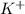 ion in the solution by using equation 1:
ion in the solution by using equation 1:
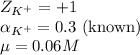
Putting values in equation 1, we get:
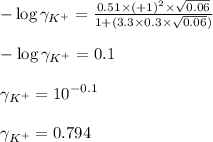
Hence, the activity coefficient of potassium ions is 0.794