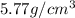Answer : The approximate density is
Explanation :
The formula used to calculate the density of the mixture is,
![\rho=[(m_A* \rho_A)+(m_B* \rho_B)+(m_C* \rho_C)]](https://img.qammunity.org/2020/formulas/chemistry/college/cogrurc6t0sicjsxj418p5zsyb460rnr71.png)
where,
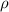 = density of mixture = ?
= density of mixture = ?
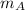 = mass of fraction of component A = 46.3 % = 0.463
= mass of fraction of component A = 46.3 % = 0.463
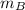 = mass of fraction of component B = 23.15 % = 0.2315
= mass of fraction of component B = 23.15 % = 0.2315
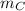 = mass of fraction of component C = 30.55 % = 0.3055
= mass of fraction of component C = 30.55 % = 0.3055
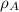 = density of component A =
= density of component A =
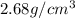
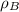 = density of component B =
= density of component B =
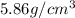
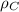 = density of component C =
= density of component C =
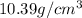
Now put all the given values in the above formula, we get:
![\rho=[(0.463* 2.68)+(0.2315* 5.86)+(0.3055* 10.39)]](https://img.qammunity.org/2020/formulas/chemistry/college/lfr8wk4iqgqbk6yiw27ghfr498qzo7dgz1.png)
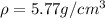
Therefore, the approximate density is