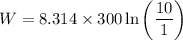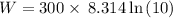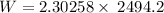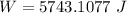Answer:
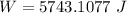
Step-by-step explanation:
The expression for the work done is:
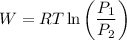
Where,
W is the amount of work done by the gas
R is Gas constant having value = 8.314 J / K mol
T is the temperature
P₁ is the initial pressure
P₂ is the final pressure
Given that:
T = 300 K
P₁ = 10 bar
P₂ = 1 bar
Applying in the equation as: