Answer: The molar volume of the Argon is 0.321 L/mol and compression factor is 0.658
Step-by-step explanation:
To calculate the compression factor, we use the Virial equation to the second order, which is:
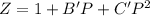
where,
Z = compression factor
B' = second virial constant =
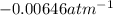 (From standard values)
(From standard values)
C' = third virial constant =
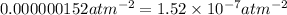 (From standard values)
(From standard values)
P = pressure of the gas = 53 atm
Putting values in above equation, we get:
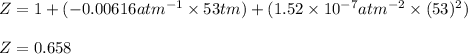
Now, calculating the molar volume, by using the equation of compression factor:
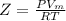
where,
Z = compression factor = 0.658
P = pressure of the gas = 53 atm
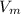 = molar volume of gas = ?
= molar volume of gas = ?
R = Gas constant =
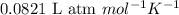
T = Temperature of the gas = 315.2 K
Putting values in above equation, we get:
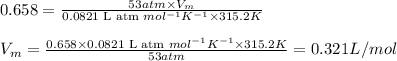
Hence, the molar volume of the Argon is 0.321 L/mol and compression factor is 0.658