Answer: The correct answer is Option a.
Step-by-step explanation:
We are given:
Mass of copper used initially = 0.325 g
When, the given amount of copper was exposed to air, copper undergoes oxidation and leads to the formation of its oxide.
The chemical equation for the formation of copper oxide follows:
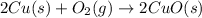
During this reaction, some of the copper gets converted to its oxide.
So, after the second day of lab, copper will be found less than the initial amount taken.
Hence, the correct answer is Option a.