Answer:
a) 70.074 of gallons of 0.05 M solution we can make.
b) 0.0834 M is the molar concentration of the resulting solution.
Step-by-step explanation:
Concentration of alum solution = c
Moles of alum in solution = n
Volume of solution = V
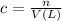
a) Mass of alum = 10 lbs = 4535.92 g
(1 lb = 453.592 g )
Molar mass of alum
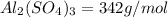
Moles of alum =
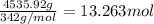
Volume of alum solution of 0.05 m = V
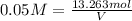
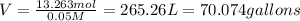
(1 L = 0.264172 gallons)
70.074 of gallons of 0.05 M solution we can make.
b) Moles of alum =
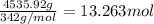
Volume of the barrel = 42 gallons = 158.99 L
Concentration of alum : C
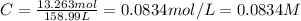
0.0834 M is the molar concentration of the resulting solution.