Answer:
The units of a are A. a = atm.s³.
Step-by-step explanation:
In the equation P = a (1/t³) + b, both terms "a (1/t³)" and "b" must have units of pressure, e.g., atm. Regarding units, we can say:
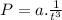
We can solve this for a:
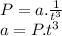
If we replace P and t with proper units, then
a = P.t³ = atm.s³