Answer:
162.2 g/mol
Step-by-step explanation:
Molar mass is defined as the mass in 1 mole of the substance. It is calculated by adding the molar mass of the substituents each multiplied by the subscript they have in the formula.
Thus, To calculate molar mass of nicotine having formula,
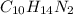
Molar mass of C = 12.0107 g/mol
Molar mass of H = 1.00784 g/mol
Molar mass of N = 14.0067 g/mol
Thus, molar mass of nicotine =
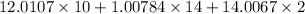 = 162.2 g/mol
= 162.2 g/mol