Answer:
8544
Step-by-step explanation:
Given:
An alternating copolymer,
thus, the number of both types of repeat units will be the same.
average weight of a poly(styrene-butadiene) = 1,350,000 g/mol
and,
there are 8 atomes of carbon and 8 atoms of hydrogen in the styrene repeat unit,
also, There are 4 atoms of carbon and six atoms of hydrogen in butadiene repeat units.
Therefore,
when the styrene-butadiene is combined the total number of carbon atoms in a molecule will be ( 8 + 4 ) i.e 12 atoms
and, the total number of Hydrogen atoms will be ( 8 + 6 ) i.e 14 atoms
Therefore, the total molecular weight will be
m = 12 × Molecular weight of the carbon + 14 × Molecular weight of the hydrogen
or
m = 12 × 12 g/mol + 14 × 1 g/mol
or
m = 158 g/mol
Now,
the average number of styrene and butadiene mer units per molecule will be
=
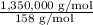
= 8544.30 ≈ 8544