Answer:
The final temperature will be 3600 K.
Step-by-step explanation:
Side 1
T=927°C
T=1200 K
Lets take volume of one side is V then the volume of other side will be 2V. The final volume of system will become 3 V.
The system is closed so the mass of system will remain constant .
We know that for ideal gas P V = m R T
The final pressure is same as initial pressure so we can say that
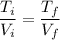
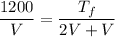
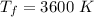
So the final temperature will be 3600 K.