Answer:
T = 288.15 K
Step-by-step explanation:
from gas law for dry air, the average surface temperature of dry air in the atmosphere can be computed as:
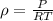
where,
 is density of dry air = 1.225 kg/m3,
is density of dry air = 1.225 kg/m3,
P is standard air pressure) =101.325 kPa = 101325 Pa,
R is gas constant = 287.05 J / (kg.K)
substituting the values, we get
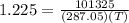
solving for T we get
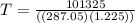
T = 288.15 K