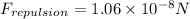Answer:
Part a)
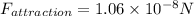
Part b)
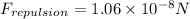
Step-by-step explanation:
Part a)
Equilibrium distance is the distance between two centers when two ions just touch each other
So here we will have
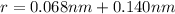
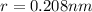
Now Force of attraction between two ions is given by Coulomb's law
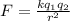
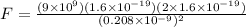
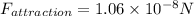
Part b)
At equilibrium separation net force between two ions must be zero
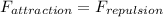
so the attraction force and repulsion force must be of equal magnitude
so we have