Answer:
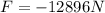 , attractive.
, attractive.
Step-by-step explanation:
For calculating this force we use the Coulomb Law:
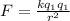
Where
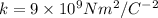 is the Coulomb's constant,
is the Coulomb's constant,
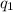 and
and
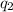 the values of each charge and r the distance between them.
the values of each charge and r the distance between them.
Since the Coulomb's constant as I wrote it is in S.I. we have to write all the magnitudes in that system of units, and substitute:
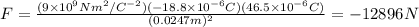
This force is attractive since both charges are of opposite sign.