Step-by-step explanation:
The given data is as follows.
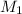 = 1.00 M,
= 1.00 M,
 = 50.0 ml
= 50.0 ml
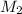 = ?,
= ?,
 = 5.75 ml
= 5.75 ml
Therefore, calculate the concentration (in M) of acetic acid as follows.
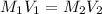
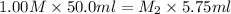
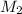 =
=
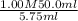
= 8.69 M
Thus, we can conclude that the concentration (in M) of acetic acid present in the given vinegar is 8.69 M.