Answer:
The volume of the bubble near the surface will be 9.47 m³
Step-by-step explanation:
Given that,
Depth = 52.0 m
Volume = 1.50 m³
Temperature at bottom = 5.5°C
Temperature at the top = 18.5°C
We need to calculate the pressure at the depth 52.0 m
The pressure is
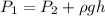
Where,
 = Pressure at the surface
= Pressure at the surface
 = Pressure at the depth
= Pressure at the depth
Put the value into the formula
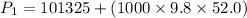

We need to calculate the volume of the bubble just before it reaches the surface
Using equation of ideal gas
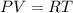
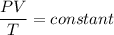
Now, The equation of at bottom and top
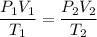
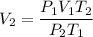
Put the value into the formula

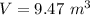
Hence, The volume of the bubble near the surface will be 9.47 m³