Answer:
a)
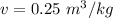
b)W= 50.4 KJ
c)Q= -10.4 KJ
It means that heat will be rejected from the system.
Step-by-step explanation:
m= 4 kg
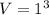
Work in put = 14 W 14 J/s
1 h = 60 sec
Work in put = 14 x 60 J
W=14 x 60 J =840 J
ΔU= 10 KJ/Kg
ΔU= 10 x 4 = 40 J
a)
We know that specific volume is the ratio of volume and mass.
v= V/m
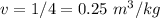
b)
Energy transfer by work =W
W=14 x 60 x 60 J =50.4 KJ
W= 50.4 KJ
c)
From first law of thermodynamics
Q = ΔU + W
Here work in added to the system that is why W= -840 J
Q = 40 - 50.4 KJ
Q= 40 - 50.4 KJ
Q= -10.4 KJ
It means that heat will be rejected from the system.