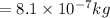Answer:
mass of the helium gas will be
Step-by-step explanation:
We have given volume of the helium
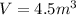
Density of helium gas
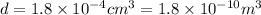
We have find the mass of the helium gas
We know that mass is given by mass = volume×density
So mass =
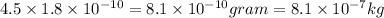
So mass of the helium gas will be