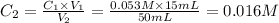Answer:
The concentration of the most dilute solution is 0.016M.
Step-by-step explanation:
First, a solution is prepared and then it undergoes two subsequent dilutions. Let us calculate initial concentration:
![[Na_(2)SO_(4)]=(moles(Na_(2)SO_(4)))/(liters(solution)) =(mass((Na_(2)SO_(4))))/(molarmass(moles(Na_(2)SO_(4)) * 0.100L)) =(2.5316g)/(142g/mol* 0.100L ) =0.178M](https://img.qammunity.org/2020/formulas/chemistry/college/zlzc6o9x5ot53xwg5vlus6r1ldtmmixada.png)
First dilution
We can use the dilution rule:
C₁ x V₁ = C₂ x V₂
where
Ci are the concentrations
Vi are the volumes
1 and 2 refer to initial and final state, respectively.
In the first dilution,
C₁ = 0.178 M
V₁ = 15 mL
C₂ = unknown
V₂ = 50 mL
Then,
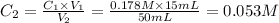
Second dilution
C₁ = 0.053 M
V₁ = 15 mL
C₂ = unknown
V₂ = 50 mL
Then,