Answer: The amount of time needed for 1 mole of electrons to pass through the lamp is 12.33 hrs.
Step-by-step explanation:
We are given:
Moles of electron = 1 mole
According to mole concept:
1 mole of an atom contains
 number of particles.
number of particles.
We know that:
Charge on 1 electron =
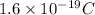
Charge on 1 mole of electrons =
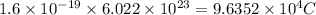
To calculate the time required, we use the equation:
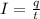
where,
I = current passed = 2.17 A
q = total charge =
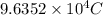
t = time required = ?
Putting values in above equation, we get:
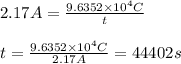
Converting this into hours, we use the conversion factor:
1 hr = 3600 seconds
So,
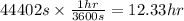
Hence, the amount of time needed for 1 mole of electrons to pass through the lamp is 12.33 hrs.