Answer:
24.02 grams of water will absorb a total of 2510 joules of energy during the temperature of the water changes from 10.0 degrees celsius to 35.0 degrees celsius.
Step-by-step explanation:
The measurement and calculation of the amounts of heat exchanged by a body or system is called calorimetry.
Between heat and temperature there is a direct proportionality relationship, where the constant of proportionality depends on the substance that constitutes the body and its mass. So, the equation that allows you to calculate heat exchanges is:
Q = c * m * ΔT
Where Q is the heat exchanged for a body of mass m, constituted by a specific heat substance c and where ΔT is the temperature variation.
In this case,
- Q=2510 J
- water has a specific heat of about 4.18
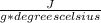
- ΔT=35.0 degrees celsius- 10.0 degrees celsius= 25.0 degrees celsius
Replacing:
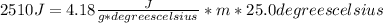
Solving you get:
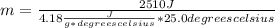
m≅ 24.02 g
24.02 grams of water will absorb a total of 2510 joules of energy during the temperature of the water changes from 10.0 degrees celsius to 35.0 degrees celsius.