Answer:
The answer to your question is: letter C, 0.1 g/ml
Step-by-step explanation:
Smallest density
density = mass / volume
a) 200 g object with a volume of 200 mL
density =
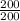 = 1 g/ ml
= 1 g/ ml
b) 100 g object with a volume of 50 mL
density =
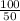 = 2 g/ ml
= 2 g/ ml
c) 1 g object with a volume of 10 mL
density =
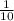 = 0.1 g/ ml
= 0.1 g/ ml
d) 10 g object with a volume of 1 mL
density =
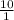 = 10 g/ml
= 10 g/ml