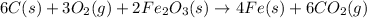Answer:
The net chemical equation for the production of iron from carbon, oxygen and iron(III) oxide.
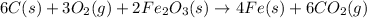
Step-by-step explanation:
Step 1: Carbon and oxygen combines together to form carbon monoxide gas.
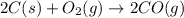 ..[1]
..[1]
Step 2: Carbon monoxide gas formed in previous step is allowed to recat with iron(III) oxide to obtain iron as a product along with carbon dioxide gas
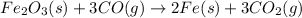 ..[2]
..[2]
The net equation of the process can be written by adding both reactions:
3 × [1 ] + 2 × [2]
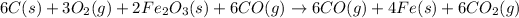
Cancelling out common compound son both sides, we get the net chemical equation for the production of iron from carbon, oxygen and iron(III) oxide.