Answer:
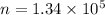
Step-by-step explanation:
It is given that,
Wavelength of the photon,
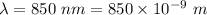
Total energy required to trip the signal,
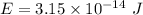
Let n is the minimum number of photons that must strike the receptor. Firsly calculating the energy of one photon as :
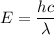
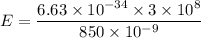
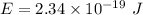
Let n is the number of photons that must strike the receptor. It can be calculated as :
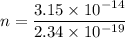
n = 134615.38
or
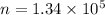
So,
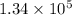 number of photons that must strike the receptor. Hence, this is the required solution.
number of photons that must strike the receptor. Hence, this is the required solution.