Answer : The molar mass of benzyl acetate is 151.25 g/mol
Explanation :
Formula used for Elevation in boiling point :
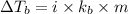
or,
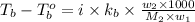
where,
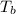 = boiling point of solution =
= boiling point of solution =
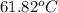
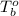 = boiling point of chloroform =
= boiling point of chloroform =
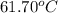
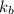 = boiling point constant of chloroform =
= boiling point constant of chloroform =
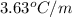
m = molality
i = Van't Hoff factor = 1 (for non-electrolyte)
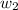 = mass of solute (Benzyl acetate) = 0.125 g
= mass of solute (Benzyl acetate) = 0.125 g
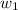 = mass of solvent (chloroform) = 25.0 g
= mass of solvent (chloroform) = 25.0 g
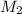 = molar mass of solute (Benzyl acetate) = ?
= molar mass of solute (Benzyl acetate) = ?
Now put all the given values in the above formula, we get:
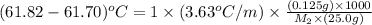
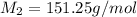
Therefore, the molar mass of benzyl acetate is 151.25 g/mol